An atom is constituyed by particles called protons, neutrons and electrons.
- Protons: Is a small positively charged particle and it's located in the nucleus of the atom.
- Neutrons: They have no electrical charge and they are also located in the nucleus of the atom.
- Electrons: Tiny particles, negatively charged. They move around the nucleus of the atom.
#Fact: Electrons occupy most of the atom's volume, though not much of its mass.
#Fact: It takes almost 2,000 electrons to equal the mass of just ONE proton.
#FunFact: Because atoms are too small to be measured in everyday units, scientists use units known as atomic mass units (amu).
#Fact: An electron's mass is about 1/2,000 amu.
Isotopes
Atoms with the same number of protons, but a different number of neutrons.
You can identify an element by its number of protons in the nucleus. The atomic number is the number of protons in its nucleus, and each element has a unique one. The mass number is the sum of the protons and neutrons in the atom's nucleus.
Models of Atoms
It took over two centuries for scientist to creat the model of an atom in an effort to understand why matter behaves as it does. Check out how it has changed through all these years:
Dalton concluded that each element is made of atoms that are all alike. He also thought that every element have atoms of different mass. He imagined atoms as solid balls. Just like the ones on billiards!
Just 89 years later, J.J. Thomson discovered the electrons. He suggested that an atoms is a positively charged sphere with electrons with electrons in it. A chocolate chip cookie may be a good way to describe Thomson's model!
Nagaoka Model - 1904

Now, it just took the Japanese physicist, Hantaro Nagaoka, 7 years after Thomson's Model to make a new model. He proposed that the atom's sphere was positively charged and the electron were revolving around it. Just like the planets around the sun!
Rutherford Model - 1911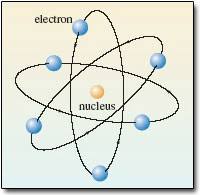
It didn't take more than 7 years for british physicist Ernest Rutherford to prove all these last models wrong! He concluded that the atom is mostly empty space and that electrons orbit randomly around the nucleus (that's positively charged).
Bohr Model - 1913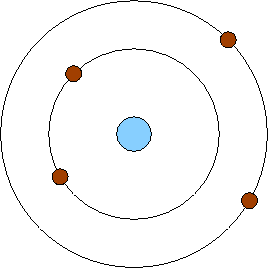

Two years after Rutherford's amazing conclusion, Danish physicist Niels Bohr proposed that electrons moved in specific layers, rather than randomly. He also argued that atoms absorb or give off energy when the electrons move from one layer to another.
Chadwick Model - 1932 

After 19 years, British physicist James Chadwick came up with the discovery of the neutron. Neutrons are the reason of why atoms were heavier than the total mass of just their protons and electrons.
The Present Modern Model
It results to work done from the 1920s to the present. Electrons are like in a negatively charged cloud around the nucleus. #Note: It's impossible to determine where is an electron at a given time.
Atoms are amazingly small.
#AmazingFact: The smallest visible speck of dust may contain 10 million billion atoms!
#AmazingFact: A sheet of paper is about a million atoms thick!
Scientists create models to describe atoms becuase they are too small. Could be diagrams, mental pictures, mathematical statements, or an object may help to describe ideas about the natural world.
For example:
A Blog. The people writting their everyday blog are the nucleus, the ones who give the energy or attraction for other people to stick around. People who read them are like electrons. Suck up the energy, in this case the information of what they are reading!
Really? Was it too hard to describe an atom than it took them over two centuries?! Well, they didn't have blogs, right? LOL
Finally, here's a video of why Bohr's Model explain many things about the structure of an atom and the behave of its electrons.
How cool!



